Ceramics may be crystalline or non crystalline and are strong stiff brittle chemically inert and do not conduct heat or electricity but properties vary widely.
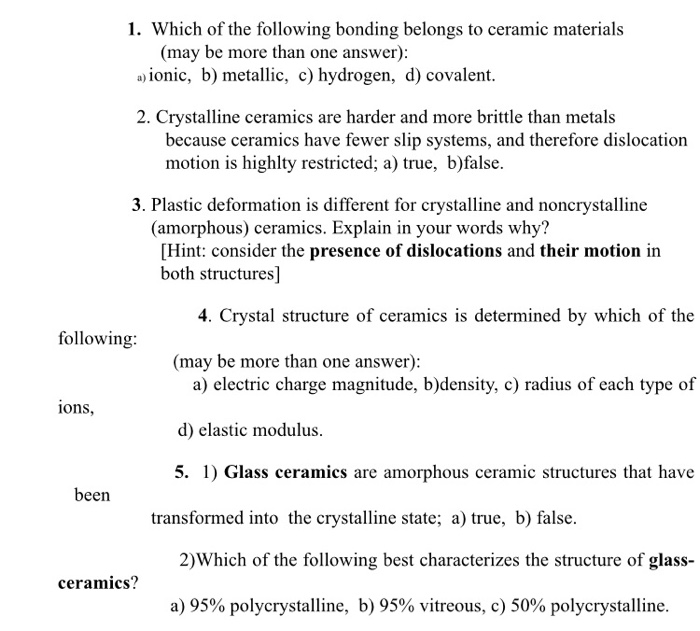
Explain why ceramics tend to be strong but brittle.
Ceramics tend to be strong but brittle because of the ionic bonding present between the metal and non metal components of the material.
Ceramic materials are inorganic non metallic materials made from compounds of a metal and a non metal.
Polymers are strong and tough and often flexible.
Take a look at the following video showing schematically how a crack in a metal becomes a blunted crack and a void which can effectively stop the.
Why can metals be scratched and develop cracks and yet not catastrophically fail.
Plates tend to break if dropped onto the floor.
Ceramics are hard and strong but brittle.
The reason is that metals can slide along slip planes to break the crack up.
When subjected to a tensile load ceramics unlike metals are unable to yield and relieve the stress.
Diamond is hard because it has a much more rigid crystalline structure.
Hard wear resistant brittle refractory thermal insulators.
Ionic bonds are very strong and require a relatively large.
Due to ceramic materials wide range of properties they are used for a multitude of applications.
They withstand chemical erosion that occurs in other materials subjected to acidic or caustic environments.
Ceramics hard brittle materials.
For ceramics this type of transition occurs at much higher temperatures than for metals.
Like bricks it is hard and strong but also brittle.
In general most ceramics are.
Ceramics tend to be weak in tension but strong in compression.
This explains why ceramics are both hard and brittle.
A ceramic material is an inorganic non metallic often crystalline oxide nitride or carbide material.
So why are ceramics brittle.
The impact energy needed for fracture drops suddenly over a relatively narrow temperature range temperature of the ductile to brittle transition.
Graphite is soft because it s made of layers of carbon atoms that will slide and shear that s why a graphite pencil leaves lines on paper.
The discrepancy between tensile and compressive strengths is in part due to the brittle nature of ceramics.
Some elements such as carbon or silicon may be considered ceramics ceramic materials are brittle hard strong in compression and weak in shearing and tension.